SAS Certified Clinical Trials Programmer Using SAS 9 A00-280 Exam Questions
The following question will ask you to provide a line of missing code.
Given the following data set work.vs:
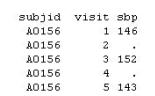
The following SAS program is submitted to create a new data set that carries forward the previous value of sbp when the value is missing.

In the space below, enter the line of code that completes the program (Case is ignored. Do not add leading or trailing spaces to your answer.).

See Below Explanation:
Answer : A
Where would you store a value collected on a case report form but not defined in an SDTM domain?
Answer : C
Which statement correctly adds a label to the data set?
Answer : D
This question will ask you to provide a line of missing code.
The following SAS program is submitted:

Which macro call prints all records from each dataset in library DB?
Answer : B
Given the following output from the TTEST Procedure: Variable:
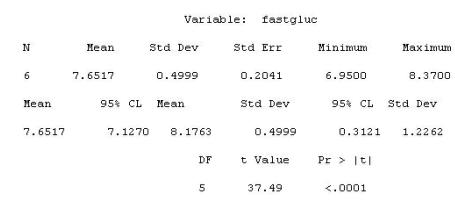
What is the t-test p-value?
Answer : B
A SAS program is submitted and the following log is written.

What is the cause of this error message?
Answer : D
This question will ask you to provide a line of missing code.
The following SAS program is submitted:

In the space below, enter the statement that completes the program correctly (Case is ignored. Do not add leading or trailing spaces to your answer.).

A.
DEFINESITE/ORDERNOPRINT;,DEFINESITE/NOPRINTORDER;DEFINESITE/NOPRINTORDER;,DEFINESITE/ORDERNOPRINT;,DEFINESITE/NOPRINTORDER;,DEFINESITE/ORDERNOPRINT;,DEFINESITE/NOPRINTORDER;,DEFINE
Answer : B