SAS Clinical Trials Programming Using SAS 9 Accelerated Version A00-281 Exam Questions
Which LIBNAME statement is valid?
Answer : D
The following SAS program is submitted:
proc sort data=SASUSER.VISIT out=PSORT;
by code descending date cost;
run;
Which statement is true regarding the submitted program?
Answer : B
Which statement assigns the current date to the character variable CURRDT?
Answer : A
This question will ask you to provide a line of missing code. The following SAS program is submitted:
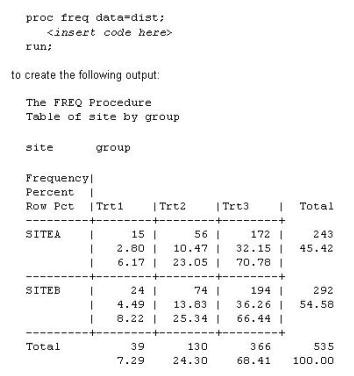
Which statement is required to produce this output?
Answer : A
This question will ask you to provide a line of missing code. Given the following data set LABS(only first 10 lines shown):
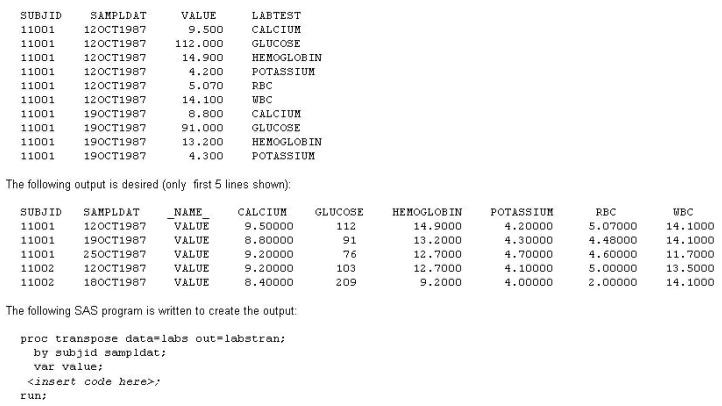
In the space below, enter the statement that completes the program to produce the desired output (Case is ignored. Do not add leading or trailing spaces to your answer.).

See Below Explanation:
Answer : A
Given the data set HE:
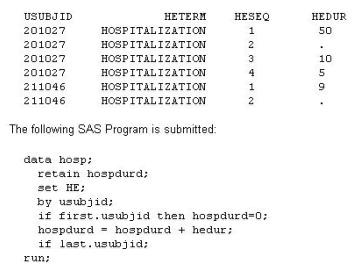
What will the values be of variable HOSPDURD for the two subjects?
Answer : B
Review the following procedure format:

What is the required type of data for the variable in this procedure?
Answer : B